By: Maeve Brady
Chemistry, and just science in general, correlates with everything in our lives. Now, something that directly relates to the water we drink, the air we breathe, and the DNA we contain is covalent bonds or also known as covalent bonding. Now, after hearing about this, you may be wondering, what exactly is covalent bonding? Well, after the further analysis I will provide in this article, I hope you gain a better understanding about its properties and how it appears in our everyday lives.
A covalent bond is a common occurrence in our lives. In simpler terms, according to the distinguished researchers at the University of Hawai’i at Mānoa, “The puppies represent atoms. The bones represent one of their electrons. Both puppies share both bones” Covalent bonding is a phenomenon where elements may share electrons in order to fill their valence electrons to get a full set. This is unlike ionic bonding, where elements steal electrons instead of sharing electrons. Covalent bonds have the goal of sharing their valence electrons to completion.
For context, in the periodic table, all of the elements have valence electrons who are located in the outermost electron shell of an atom. According to its properties, every element wants to become more stable by gaining a full set of eight valence electrons, this minimizes their energy usage and with this full set, or close to full set, of valence electrons, the element becomes less reactive. In certain circumstances, depending on the group in the periodic table, we may see different results in how reactive they are. You can check what group they are in.
If an element is in group five, then it has five electrons existing in it. And if it is in group sixteen, then it has six electrons, not sixteen. So, in the case of group eighteen, these elements are known as “noble gases”, and they have a complete set of eight valence electrons, meaning they have a full set and are not reactive; they don’t have a reason to bond with different groups in the periodic table besides themselves.
But there are a couple exceptions to this principle. One example is Helium. Helium is a noble gas with only two valence electrons instead of eight. This is because helium’s single electron shell is already completely filled. Therefore, it still demonstrates this stable and sparse reactivity.
The aspect of covalent bonding is the formation of a complete set of valence electrons after two elements share the adequate amount of electrons with one another. And with this type of bonding there are subcategories of polar and nonpolar covalent bonding.
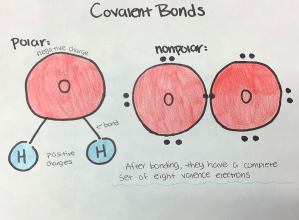
An example of polar covalent bonding would be the bonding of two hydrogen atoms and an oxygen molecule, the chemical structure of water. Oxygen has six valence electrons while each hydrogen molecule has one. This means that oxygen is almost stable and only needs two more electrons for full completion. And this is where the word “polar” comes in. “Polar” is essentially this sense of having polarity, and in the context of covalent bonding, it means a non-uniformed and unequal way of bonding. So, in the case of the chemical formation of the water molecule, oxygen and the hydrogen atoms both share their electrons, which gives them both a full set of valence electrons. But because oxygen has a higher electronegativity-oxygen has the tendency to attract electrons towards itself-so it tends to “hog” the valence electrons, hence, creating this negative charge to the originally neutral element. And with this, it creates these positive charges to the two hydrogen atoms.
Now, on the other hand, there is nonpolar bonding. Nonpolar bonding is described as the more uniformed way of bonding and in the context of covalent bonding, it is when two elements equally share valence electrons. Now, in general, you may come across more nonpolar bonds between the same elements, but that is not always the case. In fact, nonpolar bonding can be executed between two different elements as long as they share valence electrons relatively equally. The most prominent example of nonpolar covalent bonding is when two oxygen atoms can each contribute two valence electrons to complete the others’ set.
Now for the big question. How can we find Covalent Bonding in our everyday lives? Well, that is simple to answer. If we truly analyze it, we can tell that covalent bonds are in more than we may have initially thought. For example, one of the most popular examples is the properties H2O. According to the distinguished researchers at the University of Hawai’i at Mānoa, “The unequal sharing of electrons between the atoms and the unsymmetrical shape of the molecule means that a water molecule has two poles-a positive charge on the hydrogen pole (side) and a negative charge on the oxygen pole (side). We say that the water molecule is electrically polar.” This demonstrates that a polar covalent bond is how H20 comes about. And we use water in our everyday lives. It is the basis of everything, and something we rely on so heavily. Besides water, covalent bonds also may be found in our air and more, as mentioned in the first paragraph.
Thank you so much for following me along as I explain the basics on covalent bonding! I hope you now have a better understanding of this process and how it appears in our everyday lives.